< Back
Intensifier Control
HiCAM Fluo overcomes high-speed fluorescence imaging challenge in microfluidics
A challenge that occurs in many different research areas is the point where a process with low luminescence has to be captured at high-speed. The HiCAM Fluo is a camera that has proved to be able to overcome this challenge in a study performed by the Max Planck institute. Jahnke, Weiss, Frey et al. (2019) published an article in which they released an alternative method for the functionalization of microfluidic droplets by self-assembly of cholesterol-tagged DNA at the droplet periphery (figure 1). In their research the kinetic interaction between the cholesterol-tagged DNA and the periphery of the droplet was studied by recording videos of the generation of these droplets
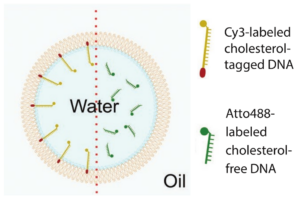
Figure 1. Schematic illustration ofa water-in-oil droplet. Left of the red dotted line the droplet is functionalized by cholesterol-tagged DNA. Right of the dotted line cholesterol-free DNA is added but the droplet is not functionalized (Jahnke et al. 2019).
“Often one cannot simply increase the amount of illumination when observing a light emitting process. The limitation can be light generation itself or the ‘inflicted’ energy might even kill your sample. The solution is to amplify the emitted light to a level that will be detected by the sensor.”
— Jeroen Wehmeijer, Business Development Manager at Lambert Instruments
In the research two challenges meet and form one extreme challenge. First of all, the process of generating droplets occurs very fast. One single droplet is generated in less than 0.003 second. In order to create a series of images of the formation of one single droplet, several frames should be captured within the time that one single droplet is formed. Let’s assume that 10 frames is the least amount of images one would like to have of the generation of one single droplet, then a frame rate of at least 3334 frames per second is required. Only high-speed cameras are capable of such high frame rates.
The second challenge is the low light intensity. In this experiment the light that is to be detected originates from a Cy3-dye. It is generally known that the light intensity of a fluorescence signal is rather low. Therefore, a very sensitive camera is required for this experiment. The sensitivity needed in this experiment originates from an image intensifier.
The combination of these two requirements makes it an extreme challenge for cameras, as it should be very sensitive at high-speed. “With conventional high-speed cameras it is difficult to image fluorescence processes because of the low intensity of the fluorescence signal” to quote Frey (comparison with conventional high-speed camera). Of course there are rather sensitive cameras, but those would be too slow to capture the generation of the droplets.
Imaging setup
The kinetic interaction between the cholesterol-tagged DNA and the periphery of the droplet was studied using the HiCAM Fluo. In figure 2 an overview of the set up is shown. The HiCAM Fluo is easily lens coupled to the camera port of the microscope, as the back focal distance of the input lens mount is already pre-set. The camera is connected to a frame grabber in a computer by a CoaXPress connection, allowing direct data streaming to the hard disc with a transfer speed up to 25 Gbps.
In order to image the Cy3-labeled DNA (λ = 550 nm) a fluorescence illuminator (HBO 100, Carl Zeiss AG, Germany) and FS43HE filter (Carl Zeiss AG, Germany) were used. The droplets were enlarged using a 40x objective (LD Plan-Neofluar 40x/0.6 Korr, Carl Zeiss AG, Germany). The photocathode determines the spectral sensitivity of an intensified camera. When fabricating the camera, the photocathode was selected based on the range of dyes expected to be used. Based on the Cy3-dye, the GaAsP photocathode was selected, as this photocathodes has the highest quantum efficiency at a wavelength of 550 nm.
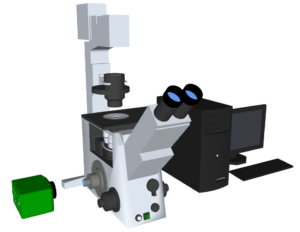
Figure 2. Simplified overview of the set-up; excisting of a HiCAM Fluo camera (green), a microscope and a computer.
Microfluidic device
In the experiment microfluidic DNA-functionalized surfactant-stabilized water-in-oil droplets were formed using a Microfluidic PDMS-based device (Sylgard 184, Dow corning, USA). In figure 3 a schematic representation of this microfluidic device is presented. The device consists of one oil inlet and one aqueous inlet. The water inlet contains 10 µM Cy3-labeled cholesterol-tagged or cholesterol-free DNA and is set to a volume flow rate of 30 µLh-1. The oil inlet contains commercial surfactant and is set to a volume flow rate of 120 µLh-1. The oil inlet splits up into two oil phases. The aqueous inlet passes into a single aqueous phase. These three phases come together in flow-focusing cross junction. At the flow-focusing cross junction water droplets in oil are generated and the droplets flow to the outlet.
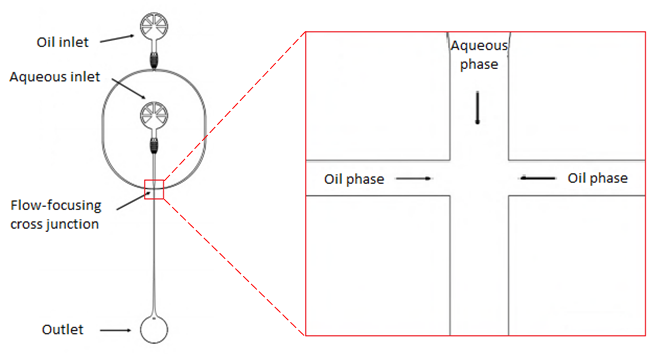
Figure 3. Schematic overview of a micro fluidic PDMS-based device. At the left side an overview ofthe device is shown, starting at the top with an oil inlet which splits up into two oil phases. The aqueous inlet passes into a single aqueous phase. These three phases come together in flow-focusing cross junction, shown enlarged on the right side. At the flow-focusing cross junction water droplets in oil are generated and flow to the outlet (Jahnke et al. 2019).
Camera settings
In the experiment three videos are captured: One at the flow-focusing cross junction and two at the outlet. In order to be able to capture the generation of one single droplet, a frame rate of 4086 frames per second was used for the first video. The cost of this high frame rate is a down scale of the resolution to 640 x 480 pixels.
For the second and third video a lower frame rate of 1000 frames per second was enough. Although the researcher kept the resolution at 640 x 480 pixels, the HiCAM Fluo is capable to run at 1000 frames per second at its full resolution of 1280 x 1024 pixels. With these settings the required high-speed is met.
The intensifier of the HiCAM Fluo played a crucial role in meeting the required high sensitivity. In this experiment a single stage intensifier with dual MCP was placed in the HiCAM Fluo. During the experiment the MCP gain was set to a gate width of4 ms and MCP gain of 1.1 kV.
Results
In order to be able to study the kinetics between the cholesterol-tagged DNA and the periphery of the droplet, three videos were captured by the HiCAM Fluo. The first video was captured at the flow-focusing cross junction.
A series of 12 images were captured of the generation of one single droplet. Below the last 8 images ofthe series is shown. At the bottom of each image, the three phases come together. The two oil phases are black, while it is not fluorescently marked. The water phase contains the Cy3-labeled cholesterol-tagged DNA and are therefore detected as white. At the last image the droplet is totally surrounded by the oil. Both the droplet and aqueous phase are homogenous white in the video. So, the cholesterol-tagged DNA is homogenously spread throughout the droplet, at the moment the droplet is generated.
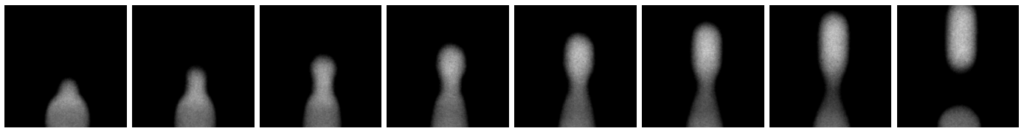
Figure 4. Last 8 images out of a series of 12 images (Jahnke, Weiss, Frey et al; 2019): Generation of one single droplet (0.003 s) containing Cy3-labeled DNA, captured with a frame rate of 4086 frames per second.
In figure 5 a screenshot of video 2 is shown. Video 2 is captured at the output, using Cy3-labeled cholesterol-tagged DNA at the aqueous inlet. A droplet is shown as a white circle. Looking closely at the image of a droplet, the white appears brighter at the edges of the circle. The brightness at the edges of the circle is caused by an increase of concentration Cy3-labeled cholesterol-tagged DNA at the droplet’s periphery.
In figure 6 a screenshot of video 3 is shown. Video 3 is also captured at the output, but now the aqueous inlet contained Cy3-labeled cholesterol-free DNA. Again the aqueous droplets are imaged as white circles. In this case the droplet appears homogenous white, which means that the cholesterol-free DNA is evenly spread throughout the whole droplet.

Figure 5. Droplets at the output, containing Cy3-labeled cholesterol-tagged DNA. The concentration DNA at the periphery is increased.
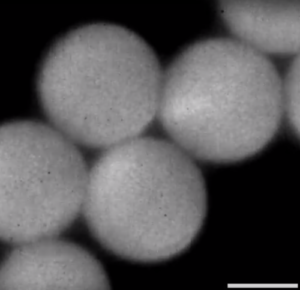
Figure 6. Droplets at the output, containing Cy3-labeled cholesterol-free DNA. The DNA is homogeneously spread throughout the droplet.
Videos 1 and 2 prove that this process takes place in the time the droplets flow from the focusing junction to the outlet. The time this process take can be calculated based on the flow rate and the dimensions of the microfluidic device (30 µm x 30 µm x 7 mm):
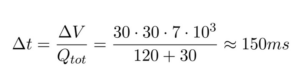
Results using a conventional high-speed camera
Using a conventional high-speed camera in this experiment, the challenge of capturing low light intensities at such a high frame rate could not be overcome. “It is just not possible to image the fluorescence dye at such low concentration under such high production frequencies with a conventional high-speed camera. The intensity from the fluorescent signal (excited photons) is not strong enough for the sensor of a conventional high-speed camera, therefore we used the HiCAM Fluo.” to quote Frey. In order to be able to detect the light with a conventional high-speed camera, the exposure time has to be increased. To be able to increase the exposure time, the frame rate should be lowered. Lowering the frame rate would mean that the formation of the droplet could not be detected anymore, as there are not enough images captured per generation of one single droplet. The only way to make the generation of droplets visible with a conventional camera is by reducing the flow rate of the oil and water inlet of the microfluidic device. And thereby changing the challenge instead of overcoming it.
Conclusion
In this application note the performance of the HiCAM Fluo was presented, based on an experiment performed by the Max Planck Institute. The experiment formed an extreme challenge for a camera as it should be able to record a process with low luminescence at high-speed. Although conventional high-speed cameras were capable of the high frame rate, they lacked the high sensitivity and therefore could not overcome this challenge. The HiCAM Fluo has proved to be able to overcome this extreme challenge; having the high frame rate due to its integrated high-speed camera and the extreme sensitivity due to its integrated single-stage dual MCP intensifier.
References
Jahnke, K., Weiss, M., Frey, C., Antona, S., Janiesch, J., Platzman, I., Göpfrich, K., & Spatz, J.P. (2019). Programmable Functionalization ofSurfactant-Stabilized Microfluidic Droplets via DNA-Tags. Advanced Functional Materials, 29(23).


