< Back
Intensifier Control
Frequency-Domain FLIM for Beginners
Fluorescence lifetime imaging microscopy (FLIM) can be performed in the time domain and in the frequency domain. Scanning single point lifetime detection units on confocal laser scanning microscopes mainly operate in the time domain. Camera-based lifetime detection on widefield, multi-beam confocal and total internal reflection fluorescence (TIRF) microscopes operate both in time domain and frequency domain. The Lambert Instruments LIFA for example is a fast frequency-domain system, whereas the Lambert Instruments TRiCAM can be operated both in the frequency and time domains.
Time Domain
In the time domain the fluorescence decay can be measured by using time-correlated single photon counting (TCSPC) or fast-gated image intensifiers. A measurement requires short excitation pulses of high intensity and fast detection circuits. Each point in the sample is excited sequentially. TCSPC records a histogram of photon arrival times at each spatial location using Photo Multiplier Tubes (PMTs) or comparable single photon counting detectors. Fast-gated image intensifiers measure fluorescence intensity in a series of different time windows. With both time domain techniques lifetimes are derived from exponential fits to the decay data. When sufficient channels (time windows) are used, multi-exponential lifetimes can be extracted.
Fluorescence lifetime imaging microscopy (FLIM) can be performed in the time domain and in the frequency domain. Scanning single point lifetime detection units on confocal laser scanning microscopes mainly operate in the time domain. Camera-based lifetime detection on widefield, multi-beam confocal and total internal reflection fluorescence (TIRF) microscopes operate both in time domain and frequency domain. The Lambert Instruments LIFA for example is a fast frequency-domain system, whereas the Lambert Instruments TRiCAM can be operated both in the frequency and time domains.
Time Domain
In the time domain the fluorescence decay can be measured by using time-correlated single photon counting (TCSPC) or fast-gated image intensifiers. A measurement requires short excitation pulses of high intensity and fast detection circuits. Each point in the sample is excited sequentially. TCSPC records a histogram of photon arrival times at each spatial location using Photo Multiplier Tubes (PMTs) or comparable single photon counting detectors. Fast-gated image intensifiers measure fluorescence intensity in a series of different time windows. With both time domain techniques lifetimes are derived from exponential fits to the decay data. When sufficient channels (time windows) are used, multi-exponential lifetimes can be extracted.
Frequency Domain
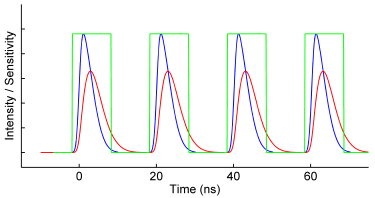
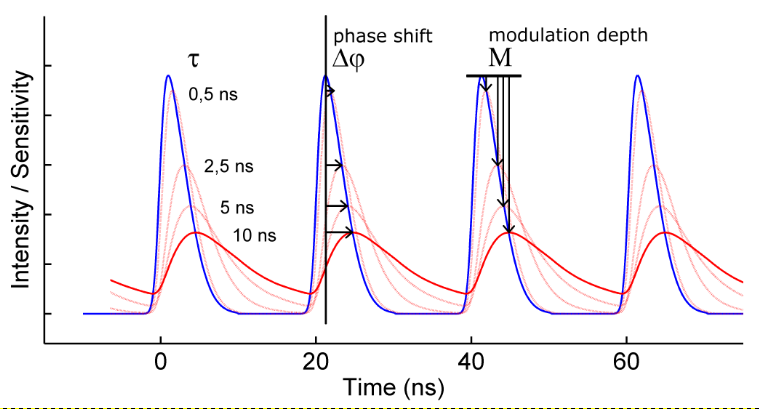
The frequency-domain FLIM technique requires a modulated light source and a modulated detector. The excitation light is modulated or pulsed in intensity at a certain radio frequency (the blue curve in the figure below). The induced fluorescence emission will mirror this modulation pattern and show, due to the fluorescence decay, a delay in time in the form of a phase-shift (the red curve). In addition, the modulation depth will decrease with respect to the excitation light, while the average intensity remains the same. The phase-shift and modulation-depth directly depend on the fluorescence lifetime and the known modulation frequency (see figure).
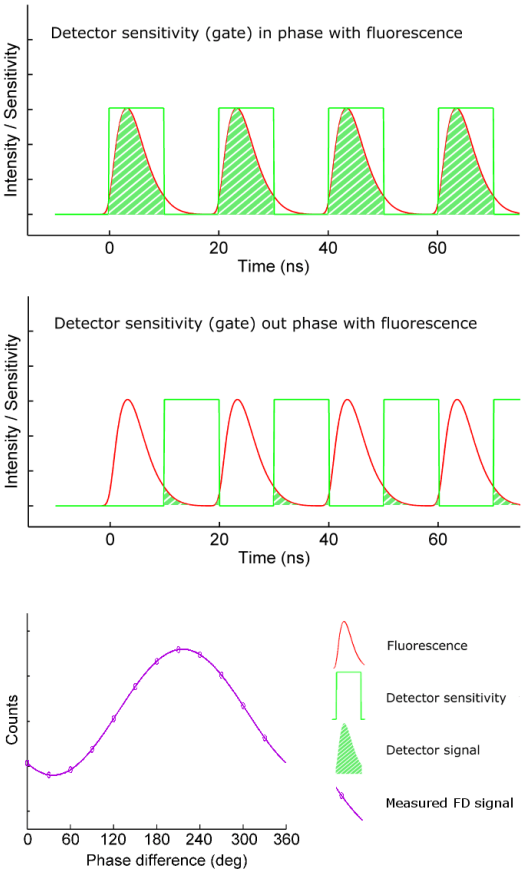
To extract the phase shift and modulation depth from the fluorescence emission signal, a homodyne detection method is often used. In this method the sensitivity of the detector – often an intensified camera – is modulated (or gated) with the same radio frequency as the light source (the green curve in the figure on the right). For a camera detector the result is an intensity image with a fixed brightness. By shifting the phase of the image intensifier with respect to the light source in a series of fixed steps a low-pass signal is generated for each pixel: the output image will be brighter or dimmer depending on whether the detector sensitivity is in or out of phase with the fluorescence emission. The result is a frequency-domain FLIM signal as a function of the phase difference between light source and camera (purple curve in the figure on the right) for each pixel in the image.
The key is that this frequency-domain signal (purple curve) exactly mirrors the phase shift and demodulation in the time domain. The phase and modulation depth can be directly extracted from the measurements and are the fundamental data in a homodyne FD FLIM measurement.
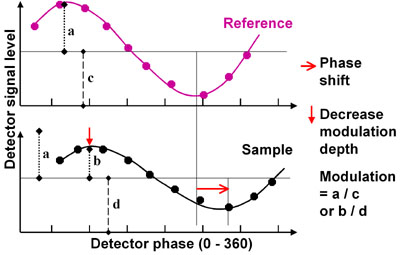
From the acquired modulation depth and phase shifts, two independent determinations of the fluorescence lifetime can be calculated. For an absolute determination the system needs to be calibrated at the pixel level with a reference fluorophore of known lifetime. For this calibration the only requirement is a FLIM acquisition of the reference fluorophore with known lifetime (figure on the right).
Multi-Exponential Decay
Some fluorophores have a multi-exponential decay, consisting of two or more lifetime components. For example, the decay of CFP is bi-exponential. These multiple lifetime components can be separated and extracted using multiple frequency measurements and the polar (or phasor) plot.
Advantages of Frequency-domain FLIM
The key advantage of frequency-domain FLIM is its fast lifetime image acquisition making it suitable for dynamic applications such as live cell research: the entire field of view is excited semi-continously – using relatively broad excitation pulses – and read out simultaneously. Hence frequency domain lifetime imaging can be near instantaneous. Another advantage of a camera-based FLIM setup, such as the Lambert Instruments LIFA, is its ease of use and its low maintenance requirements. For more information about our products, please visit the FLIM product pages and our FLIM software page.
Confocal FLIM
Confocal microscopy is a technique for high-resolution three-dimensional imaging which uses a pinhole to increase resolution in the image plane and eliminate out-of-focus light in thick specimens. The thickness of the focal plane is generally defined mostly by the objective lens and also by the optical properties of the specimen and the ambient conditions. With an imaging confocal only the light within the focal plane is detected, so that the resulting confocal images appear crisper than widefield images [read more at the cell biology wiki]. Typical applications occur within the life sciences, e.g. in cell biology. In essence there are two classes of confocal systems: single beam and multi-beam.
Confocal Scanning with CSU Spinning Disk
A Nipkow spinning disk is a multi-beam confocal scanner. The main advantage of this type of confocal imaging is the relatively fast imaging acquisition making it useful for live cell imaging applications.
The operating principle of the Yokogawa CSU spinning disk is explained here. Briefly, the disk has a spiral pattern of pinholes that is illuminated by an expanded laser beam. This generates a multi-beam illumination pattern which which the sample is illuminated. By rapidly rotating the disk this multi-beam visits all positions in the sample plane near simultaneously. The part of the fluorescence that travels back through the pinholes generates a full field confocal image at the camera detector.
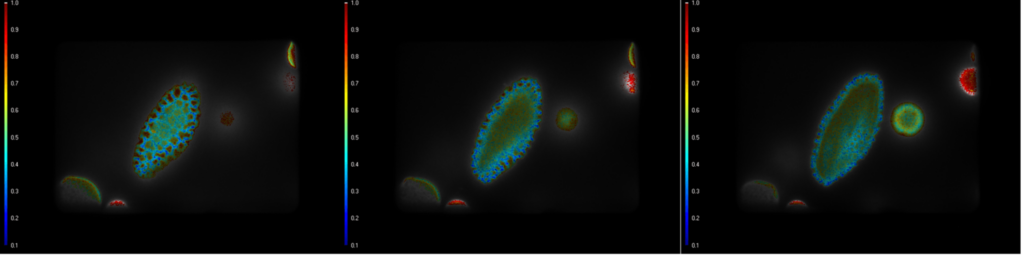
Lifetime images of mixed polled grains, at three different z-positions.
Being a camera-based system, the Lambert Instruments LIFA system for frequency domain FLIM is compatible with multi-beam confocal microscopy techniques, most notably the Yokogawa CSU spinning disk series (based on the Nipkow disk scanner), and the VTInfinity series by Visitech International Ltd.
TIRF FLIM
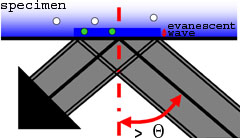
Total Internal Reflection Fluorescence (TIRF) microscopy is a super-resolution technique with high sensitivity of fluorescence near the cover glass. TIRF does not disturb cellular activity, and enables tracking of biomolecules, and the study of their dynamic activity and interactions at the molecular level. TIRF enables the selective visualisation of processes and structures of the cell membrane and pre-membrane space such as vesicle release and transport, cell adhesion, secretion, membrane protein dynamics and distribution or receptor-ligand interactions. The combination of TIRF and frequency domain FLIM makes it possible to measure fluorescence lifetimes of for instance small focal adhesions near the cover glass.
High NA TIRF objectives (up to 1.49) make it possible to introduce illumination at incident angles greater than the critical angle (θ
) resulting in TIR (Total Internal Reflection) accompanied by the formation of an evanescent wave immediately adjacent to the coverglass-specimen interface. The evanescent wave energy drops off exponentially with distance from the coverglass and reaches about a hundred nanometers into the specimen. For TIR to occur, the refractive index of the coverglass should be higher than the refractive index of the specimen (which is e.g. the case when using buffered saline solution).
The white-TIRF as well as the laser-TIRF system utilises this evanescent wave to excite fluorescent molecules in a very thin section in contact with the coverglass (here: green dots). Because the specimen is not excited beyond the evanescent wave (here: white dots), this imaging system can produce fluorescence images with an extremely high signal-to-noise (S/N) ratio and z-resolution.
For more information: Wikipedia, Olympus FluoView.
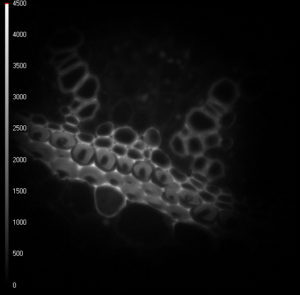
Single-Image Fluorescence Lifetime Imaging Microscopy
LIFA Flim features a unique image sensor that was designed and optimized specifically for fluorescence lifetime imaging applications. It enables lifetime imaging at unprecedented frame rates with the single-image fluorescence lifetime imaging microscopy (siFLIM) method [1].
“We used a prototype of the vTAU camera to develop a method for acquiring quantitative lifetime images from a single exposure.” says professor Kees Jalink of the Netherlands Cancer Institute. “siFLIM takes advantage of the technical capabilities of the Toggel camera and simultaneously records two 180°-phase-shifted images. This allows for video-rate lifetime imaging with minimal phototoxicity and bleaching.”
Demonstration of the Lambert Instruments Toggel camera for single-image FLIM (siFLIM) detection of histamine-induced alterations in Ca2+ concentration. Tiny …
During a single exposure, the Toggel camera records two images. The electrons in each pixel of the sensor are toggled between two storage areas at the same frequency as the modulation frequency of the light source. This results in two images that are shifted 180° in phase with respect to each other.
Because both images are recorded simultaneously, there are no artifacts of cellular movements and there is a significant improvement in the photon efficiency of the image acquisition.
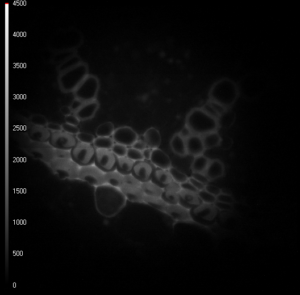
These two images of convallaria were recorded simultaneously, and because of the 180° phase shift between the two images, the recorded light intensity differs. The difference in light intensity between these two images depends on the phase shift and demodulation of the fluorescence light and can be used to calculate changes in the fluorescence lifetime of a sample [1].
[1] siFLIM: single-image frequency-domain FLIM provides fast and photon-efficient lifetime data, M. Raspe et al, Nature Methods 13, 501–504 (2016)
FLIM - the Lambert Solution
Lambert Instruments (“Lambert”), is a well-known manufacturer of high-speed, intensified and fluorescence imaging systems, with extensive experience in fluorescence lifetime imaging microscopy (FLIM). Their cutting edge FLIM systems and components are used in a broad range of research applications, including cell biology, cancer research and high throughput screening. The quality and performance of Lambert FLIM systems are trusted by researchers in the field worldwide.
Lambert FLIM systems are designed to accurately measure and analyse fluorescence lifetimes using frequency -domain techniques. Using high modulation frequencies allows for precise measurement of short fluorescence lifetimes, enabling researchers to study dynamic processes. The LIFA FLIM systems provide high lifetime resolution, single-photon sensitivity, precise timing measurements, a wide dynamic range, integration capabilities, and real-time imaging, all of which contribute to improved accuracy and versatility in fluorescence lifetime measurements.

What is FLIM?
FLIM is an acronym for Fluorescence Lifetime Imaging Microscopy. It is a technique used in fluorescence microscopy to measure the lifetimes of fluorophores, molecules that absorb light at a specific wavelength (excitation) and subsequently emit light at a longer wavelength (emission). FLIM provides information about the fluorescence lifetime of the fluorophores, the average time a fluorophore stays in an excited state before returning to the ground state and emitting light.
FLIM takes advantage of the fact that the lifetime of a fluorophore can be influenced by its microenvironment. For example, if a fluorophore is near another molecule, its lifetime may change due to energy transfer between them. FLIM measures these changes in lifetime to provide information about the local environment. By analysing the fluorescence lifetimes, FLIM can provide valuable information about various biological processes, such as protein-protein interactions, membrane dynamics, and cellular metabolism. Traditionally, FLIM has applications in fields like cell biology, neuroscience, and medical research, enabling scientists to study dynamic molecular events and gain insights into complex biological systems. However, FLIM’ s ability to rapidly provide researchers with lifetime images of their samples or study their dynamics, makes it a promising technology for areas outside the field of biology.
The Lambert Solution
Lambert’s LIFA is a camera based FLIM system. Combining excellent light sensitivity, easy image acquisition and data analysis with easy integrates into any fluorescence microscope, Lambert’s solution provides a plug-n-play experience that allows for flexibility to switch between setups quickly and easily, simplifying experiments for researchers and imaging centres. The systems are coupled with advanced tools and software for data acquisition, analysis, and visualisation of fluorescence lifetime data.
A standard Lambert LIFA system consists of a camera, light source, capture software and computer with USB connection. The camera, in combination with the light source, makes it possible to carry out frequency domain FLIM measurements. Acquisition and analysis of the FLIM data takes place through the capture software, which supports features as timelapse and multi-frequency recordings.
Utilising a fluorescence microscope, the sample of interest is illuminated with a modulated light source, such as a laser or LED. The emitted fluorescence from the sample is collected by a specialised camera. Lambert’s Capture software then takes the recorded modulated signal and calculates the phase and modulation lifetime per pixel. This data is used to generate two lifetime images, one for the phase lifetime and one for the modulation lifetime.
LIFA Cameras
The LIFA vTau camera features an image sensor with excellent temporal resolution, in the sub-nanosecond or even picosecond range. This high time resolution enables accurate measurement of short fluorescence lifetimes, which can be crucial for studying fast dynamic processes or distinguishing different fluorophores with similar emission wavelengths but different lifetimes.
Pixel resolution Readout Noise
512 x 512 px < 7.04e-6
Pixel size Framerate
16 µm 300 fps
Dark current
0.0154 /s
LIFA TRiCAM is a compact intensified camera designed for applications that require low-light imaging. TRiCAM is capable of ultra-short exposures through fast gating and frequency-domain imaging. With a gated TRiCAM camera, LIFA records a series of images and automatically shifts the timing between the light pulse and the camera exposure for time-domain FLIM on Widefield microscopes
Pixel resolution Readout Noise
1920 x 1200 px < 6.5e
Pixel size Framerate
7.6 µm >100 fps
Dark current
<7 e-/s
Light Source
The Multi-LED is a versatile pulsed excitation light source which contains up to 4 LEDs that provide non-phototoxic illumination levels, have a low cost and a long economic lifespan for FLIM imaging. Seamlessly integrated with the LIFA software, available wavelengths cover the range from 360 to 640 nm.
LIFA Software
Focusing on user-friendly solutions, Lambert’s FLIM systems are designed with intuitive interfaces and easy-to-use software, making them accessible to researchers with varying levels of expertise. They are compatible with various microscope platforms and easily integrated into existing setups.
The LIFA software guides you through your FLIM experiments from start to finish. A live view from the camera makes finding the right FLIM settings easy. the software records the FLIM data and instantly calculates the fluorescence lifetime. A time-lapsed video of the sample can also be recorded to see how the lifetime changes over time. Results can be analysed as statistical data or in several visual representations including histograms, scatter plots or a phasor plot. Lambert hardware is integrated seamlessly, allowing researchers to focus on the experiment.
Configurations
Widefield Configuration
On widefield microscopes, the LIFA vTAU camera in combination with the Multi-LED offers a capable and compact FLIM solution. vTAU connects to the widefield microscope via the camera port, while the Multi-LED connects via the standard epifluorescence port, creating in all-in-one solution.
Spinning-Disk Confocal Configuration
Being a camera-based system, the Lambert Instruments LIFA system for frequency domain FLIM is compatible with multibeam confocal microscopy techniques, most notably the Yokogawa CSU spinning disk series (based on the Nipkow disk scanner) and the VTInfinity series by Visitech International.
TIRF Configuration
Total Internal Reflection Fluorescence (TIRF) microscopy facilitates extremely high contrast visualization and thereby high sensitivity of fluorescence near the cover glass. Typically, the optical section adjacent to the cover glass is about 100nm. The unique combination of TIRF and frequency-domain FLIM makes it possible to measure lifetimes of, for instance small focal adhesions near the cover glass.
Lambert History
Lambert understands the diverse requirements of FLIM applications and offers customisable solutions. Researchers can select from different camera options excitation sources, and detectors to tailor the FLIM system to their specific needs. Known for providing reliable customer support and service, they offer technical assistance, training, and ongoing support to ensure that researchers can make the most of their FLIM systems.
1992 Lambert Instruments was founded by Bert van Geest, a specialist in cooled intensifiers and very high-speed cameras for Astronomy and Scientific Markets.
Early 2000 the first LIFA (Lambert Instruments Fluorescence attachment) FLIM system was developed. The LIFA was then developed into a commercial application, which later became a turnkey product including hardware (intensifier) and software, allowing biologists to upgrade their existing fluorescence microscope, into an advanced fluorescence microscope capable of FLIM imaging.
2014 the first FLIM System with a Toggel camera based on solid-state technology without the image intensifier was developed. Lambert has over 80 units installed Worldwide.
2023 The New LIFA camera is released. The New LIFA camera is a unique product that operates in the frequency domain. The fully integrated solution combines a FLIM system with a light source, high speed detector and software. The LIFA detector has excellent temporal resolution in the sub-nanosecond or even picosecond range. This high time resolution enables accurate measurement of short fluorescence lifetimes.
About the Author
Johan Herz, MSc Biomedical Engineering, Business Development Manager at Lambert Instruments. Johan joined Lambert as an intern during his final year at university in 2011 and later became the Specialist Service Engineer at Lambert for the LIFA Fluorescence Lifetime Imaging Microscopy (FLIM) system.
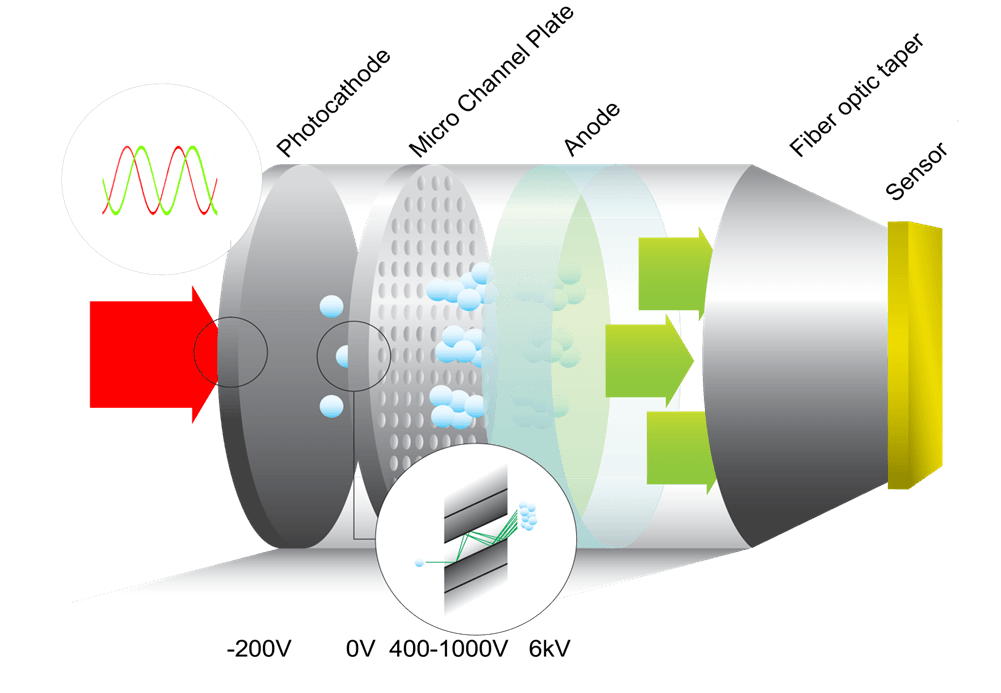
Modulated Intensifiers for Lifetime Imaging
As part of its portfolio, Lambert Instruments designs and manufactures ICCD cameras based on modulated image intensifiers for frequency domain imaging techniques such as FLIM. Standard products such as the new TRiCAM M ICCD camera, the TRiCATT M modulated intensifier attachment, and the LIFA system are all based on a proximity-focused modulated Gen II or Gen III image intensifier.
The modulated sensitivity of an image intensifier is produced by high-frequency switching of its photocathode voltage. In this modulation mode the temporal and also the spatial characteristics of the image intensifier are different from the nominal characteristics under continuous operation.
The following table lists the nominal characteristics for the Gen II and Gen III intensifiers provided.
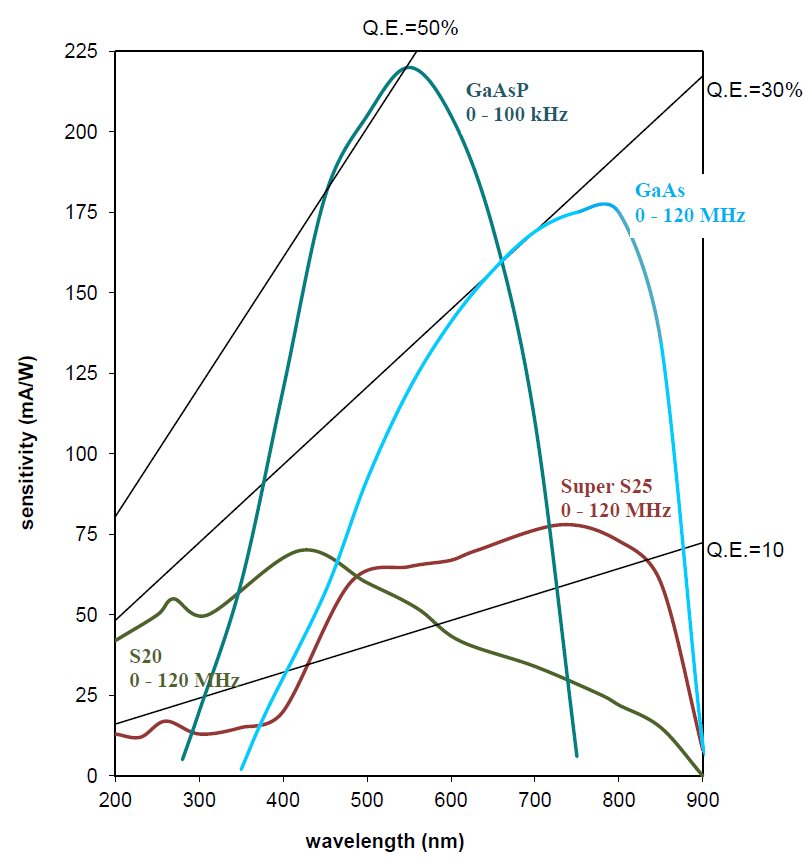
GEN II S20 & S25
GEN III GAAS
GEN III GAASP
Spatial resolution
Photocathode QE at 550 nm
28 lp/mm
14%
17 lp/mm
28%
7 lp/mm
48%
The increased sensitivity of the Gen III image intensifier results in a better lifetime accuracy, although the amount further depends on the model and the modulated light source used. For a LIFA Gen III GaAs compared to a LIFA Gen II S25 at 550nm, both using the same Multi-LED light source, the increased sensitivity results in an increased lifetime accuracy of 40% at the same acquisition speed and fluorescence intensity.
For phosphorescence lifetime imaging with the TRiCAM GM and the LIFA-P the image intensifier is operated at lower frequencies spatial resolution is higher, reaching values in excess of 35 lp/mm for a Gen III GaAs image intensifier.
Please note that the characteristics of an image intensifier are unique and are known to vary between units.