< Back
Intensifier Control
Forster Resonance Energy Transfer
Forster Resonance Energy Transfer (FRET) is the non-radiative transfer of energy from a molecule in the excited state (donor) to a molecule in the ground state (acceptor). A fluorescent donor molecule can return to the ground state by losing its energy through emission of a photon (fluorescence), or by transferring its energy to a nearby (1 – 9nm) acceptor molecule (FRET). Compared to a molecule that exhibits no FRET, the donor has more options to lose its energy. Therefore, it returns faster to the ground state, which decreases its lifetime.
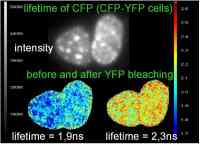
FRET is a useful tool to quantify molecular dynamics like interactions of two fluorophores by microscopy. The proteins under investigation are labelled with donor fluorophores or acceptor fluorophores. Interaction between the two fluorophores is accompanied by direct energy transfer from donor to acceptor (FRET). When FRET occurs, it means that the two proteins of interest are in such close proximity that they can interact with each other.
During FRET, a quantum of energy is transferred from a donor fluorophore to an acceptor fluorophore in a nonradiative process. So, in case of no FRET, the donor fluorophore is excited and emits photons. The acceptor fluorophore does not emit photons, because it is not excited. In case of FRET, the donor fluorophore is excited, but in stead of emitting all its energy as photons, it transfers some of its energy to the acceptor fluorophore that becomes excited and emits light.
Summarising, in case of no FRET only the donor fluorophore emits photons, and in case of FRET both donor and acceptor emit photons.
FRET ONLY OCCURS IF…
The donor fluorescence emission spectrum overlaps with the acceptor absorbance.
The donor and acceptor fluorophores are in close proximity (i.e. 1 – 9nm, which is at the scale of protein size).
The transition dipole moments of the donor and acceptor fluorophores are not perpendicular.
FRET PAIRS
To let FRET occur, the emission spectrum of the donor fluorophore has to overlap the excitation spectrum (absorbance) of the acceptor fluorophore. Some examples are BFP-YFP, CFP-YFP, GFP-DsRed, GFP-Cy3, GFP-mOrange, YFP-RFP, and Cy3-Cy5.
Browser based calculator to find the critical distance and FRET efficiency with known spectral overlap.


